iR Compensation in DC Corrosion Measurements
When performing DC corrosion experiments, accurate potential control is critical. One challenge that arises in these measurements is the iR-drop—a voltage loss caused by the flow of current (I) through the resistance (R) of the electrolyte. This voltage drop can distort your data and lead to misinterpretation of corrosion behavior if not properly addressed.
Understanding iR-Drop in Corrosion Cells
In any electrochemical cell, applying current between electrodes in a conductive solution creates regions of varying potential. Most of the potential change happens close to the electrode surfaces due to ionic concentration gradients. However, a portion of the voltage drop also occurs across the bulk solution—this is the iR-drop caused by the cell’s internal resistance.
In corrosion studies, the key measurement is the potential of the working electrode (typically your metal specimen) relative to a reference electrode. The goal is to measure this potential accurately without the distortion caused by solution resistance.
Three-Electrode Setup and Uncompensated Resistance
Gamry potentiostats operate using a standard three-electrode configuration, which separates the roles of current flow and voltage measurement:
- Working Electrode (WE): where the corrosion process occurs
- Counter Electrode (CE): completes the circuit and carries current
- Reference Electrode (RE): measures potential without passing current
This design minimizes the effect of iR-drop—but it doesn’t eliminate it completely. The position of the reference electrode can reduce some of the potential error. Ideally, placing the RE close to the WE reduces the amount of resistance (and therefore voltage drop) between the two. However, due to physical limitations, there's always some residual resistance known as the uncompensated resistance (Ru).
Dynamic iR Compensation with Current Interrupt
To correct for this residual error, Gamry's DC Corrosion software uses a technique called current-interrupt iR-compensation.
Here’s how it works:
- After each data point is recorded, the cell current is briefly interrupted.
- When the current stops, the iR-drop vanishes instantly (since I = 0), while the electrode surface potential remains unchanged for a short period.
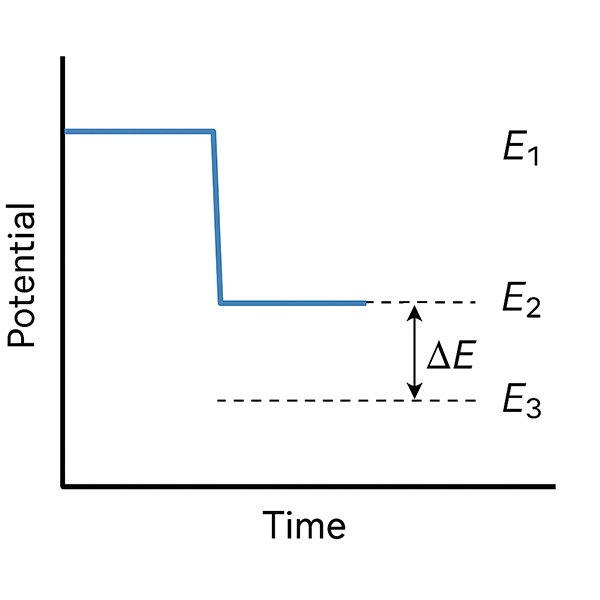
- The software records three voltages:
- E1: just before interrupt
- E2 and E3: during the current-off period
These values allow the system to calculate the true potential at the exact moment the current was turned off. This difference (ΔE) represents the uncompensated iR-drop, which is then subtracted from the measured potential for improved accuracy.
Interrupt timing is automatically adjusted based on current range:
- High current ranges: 40 µs interrupt
- Low current ranges: longer interrupt durations for stability
Why iR Compensation Matters in Corrosion Testing
Accurate iR compensation is essential for:
- Precise corrosion potential (Ecorr) and current (Icorr) measurements
- Reliable Tafel analysis and polarization resistance (Rp) results
- Minimizing artifacts in data caused by solution resistance
 Gamry’s implementation of current-interrupt iR-compensation ensures high-quality, reproducible data so you can focus on understanding corrosion mechanisms, material performance, and protective coatings.
Gamry’s implementation of current-interrupt iR-compensation ensures high-quality, reproducible data so you can focus on understanding corrosion mechanisms, material performance, and protective coatings.

