Chronoamperometry is used to study the kinetics of chemical reactions, diffusion processes (solution or films), and adsorption processes. In this technique, a potential step is applied to the electrode and the resulting current vs. time is observed. The Chronoamperometry experiment supports both single- and double-potential step experiments.
In general, before beginning the experiment, the electrode is held at a potential at which no faradaic process occurs, then the potential is stepped to a value at which a redox reaction occurs to induce adsorption, desorption, or changes in a film. Zero time is defined as the time at which the potential step is initiated. For reactions that are under diffusion-control, the current will decay with a t1/2 decay obeying the Cottrell equation:
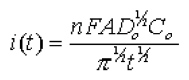
where n is the number of electrons in the redox process, F is the Faraday constant, A is electrode area, Do is the diffusion coefficient of the redox species, Co is the bulk concentration of the redox species, and t is time.
In some cases double-potential step chronoamperometry experiments are used to determine the reversibility of a reaction by comparing the results from the two potential steps.